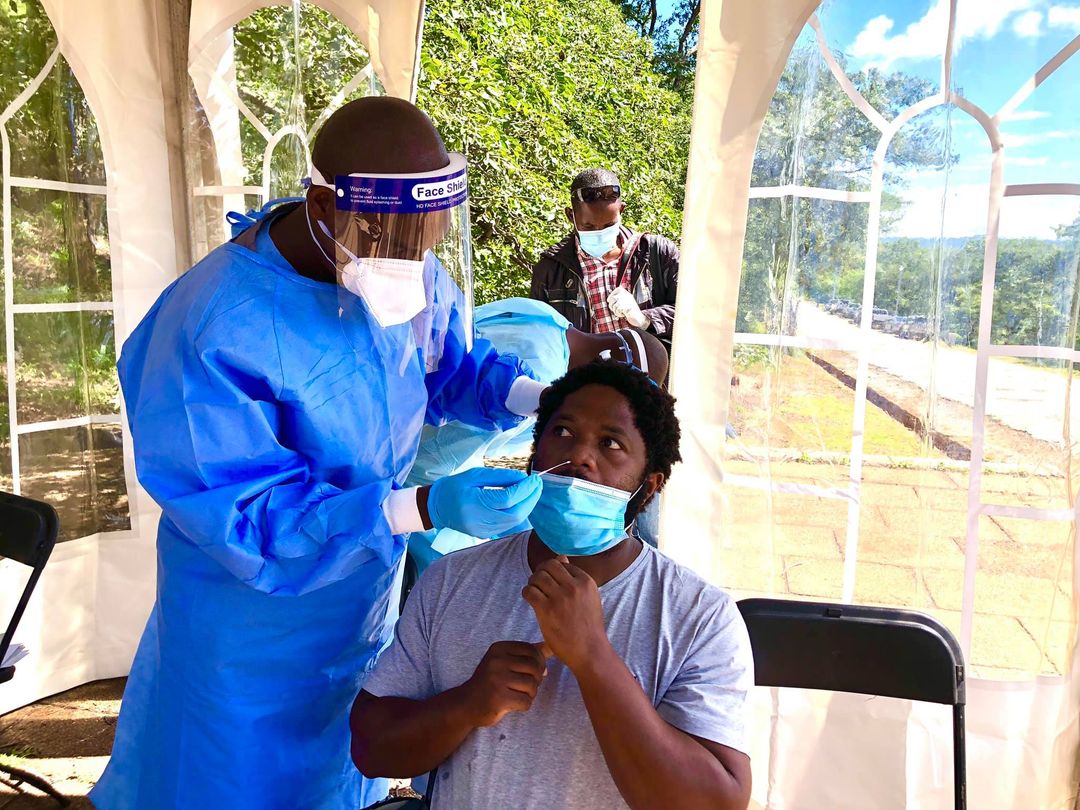
HARARE – Zimbabwe has authorised the use of Ivermectin, an anti-parasitic agent, for the treatment of Covid-19, amid wrangling among doctors over the drug’s safety and efficacy.
Seeking government approval, an association of primary care physicians touted the fusion of Ivermectin and nanosilver solution as “a game-changer” with an “excellent safety profile” and no significant side effects except “occasional dizziness and a reactive cough in a minority of patients.”
But another grouping of public health physicians raised concern and urged authorities to prohibit the drug’s administration “forthwith,” contending “there’s no sufficient evidence to support its use.”
Still, the ministry of health went ahead and issued authorisation as an “investigational Covid-19 treatment alternative” on Wednesday, saying while government had a critical obligation to protect patients, it also could not “deny them effective treatment regimes.”
“It is in this regard that authority is granted for you to proceed under Section 75 of the Medicines and Allied Substances Control Act to allow importation and use of these medicines under supervision and guidance you outlined,” said acting health secretary Robert Mudyiradima in a memo to the Medicines Control Authority of Zimbabwe.
“Ivermectin can be evaluated for both treatment and prophylaxis (prevention).”
Addressing the parliamentary health committee virtually earlier this week, Mudyiradama revealed that the drug was already being prescribed by Zimbabwe doctors albeit on a limited scale until the completion of clinical trials.
His statement came as the Zimbabwe College of Public Health Physicians (ZCPHP) pressed the government to outlaw its use, questioning the agent’s safety and efficacy on humans, and arguing that it is only licensed for animal use in Zimbabwe.
“Our conclusion is that whilst there are a number of studies of differing quality and relevance to this issue, there is no sufficient evidence to support its use,” the public health physicians said in a statement.
“We, therefore, appeal to the regulatory authorities (Ministry of Health and Child Care, Medical and Dental Practitioners Council, Medicines Control Authority of Zimbabwe and Pharmacist Council of Zimbabwe) to immediately act in the interest of public safety and stop forthwith the prescription and use of ivermectin for treatment and prevention of Covid-19.”
But the College of Primary Care Physicians of Zimbabwe (CPCPZ) pushed back in an urgent authorisation request to government saying, “We feel comfortable using this drug which has been around for 40 years, is on the World Health Organization (WHO) essential drugs list and has an excellent safety profile.”
“Since August 2020 we have adopted the use of both Ivermectin and nanosilver solution and have found this combination to be a game-changer in terms of the management of our patients,” said the primary care physicians.
“We as GPs can testify to the safety and effectiveness of using nanosilver from April 2020 to date, although we have still lost patients who have presented late in the disease.”
Without Ivermectin and nanosilver, the family doctors argued that Zimbabwe’s death toll from the deadly respiratory illness – currently at 1,122 and rising rapidly – could be significantly higher.
“After the first wave when numbers were low it was less obvious just how successful this regimen can be but when the second wave hit in mid-December we found that using this protocol was extremely effective,” they said, adding that the regimen is also minimizing reliance on auxiliary oxygen by patients.
“In fact, some practitioners estimate that they have a less than 1,5 percent mortality using this combination. We believe that the Zimbabwean case fatality rate would be significantly higher over the last month if we had not been able to use it.”
While it’s exotic to Zimbabwe, Ivermectin is approved for animal and human use in several countries, treating infections caused by parasitic worms such as intestinal strongyloidiasis and onchocerciasis, among other conditions.
Responding to a recent journal declaring it “an inhibitor” of coronavirus, the United States Food and Drug Administration (FDA) counseled against its use saying “additional testing is needed.”
Zimbabwe is currently in dialogue with Russia and China over the purchase of Covid-19 jabs while awaiting an allocation of roughly 3 million doses from the African Union (AU) in March or thereabout, authorities said this week.