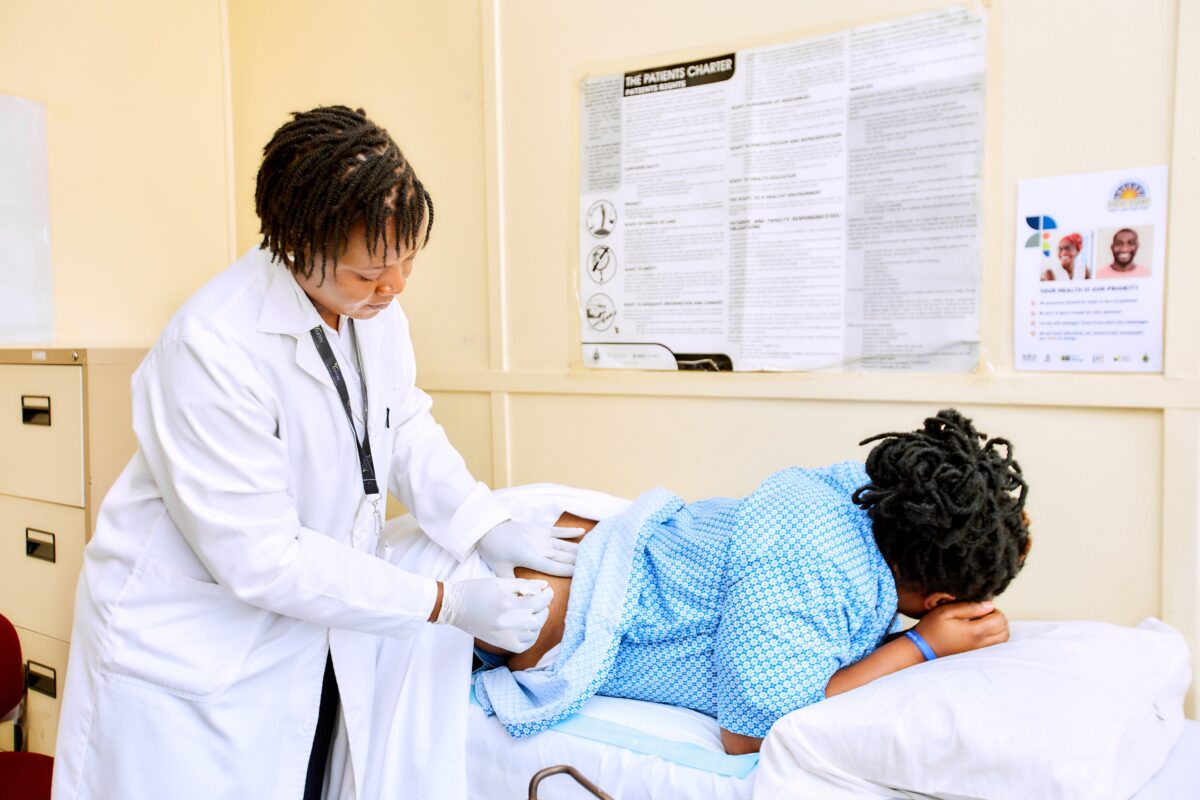
HARARE – Zimbabwe has administered the first injection of the long acting cabotegravir (CAB-LA) HIV prevention drug, which scientists hope will replace daily pills.
It is being hailed as a more convenient alternative to pre-exposure prophylaxis pills (PrEP). The injection is administered once in two months.
A woman received the injection at the New Start Centre in Harare on April 10.
In a statement, Population Solutions for Health said: “We are thrilled to have welcomed our first recipient of care for the life-changing injectable CAB-LA at New Start Centre in Harare.
“This biomedical HIV prevention option for PrEP offers a convenient and potent means of preventing HIV transmission.”
Zimbabwe became the first country in Africa to announce regulatory approval for injectable cabotegravir in November last year.
The World Health Organisation (WHO) recommends that CAB-LA may be offered to people at substantial risk of HIV infection as part of comprehensive HIV prevention approaches.
Two large studies showed that CAB-LA injections every two months were safe, well-tolerated, and highly effective in reducing the risk of HIV acquisition.
CAB-LA is the third PrEP product recommended by WHO for HIV prevention. Tenofovir-based oral PrEP was recommended in 2015 and the dapivirine vaginal ring, another long-acting product, in 2021.
Before Zimbabwe, CAB-LA had only received regulatory approval in two high-income countries. The US Food and Drug Administration (FDA) approved its use for HIV prevention in December 2021, and Australia’s Therapeutic Goods Administration (TGA) approved it in August 2022.
Zimbabwe’s fight against HIV has seen Aids-related deaths fall from an estimated 130,000 in 2002 to 20,000 in 2021.